|
Chest Pain Implications | Noninvasive Heart Transplant Monitoring
June 13, 2022
|
|
|

|
|
Together with
|

|
|
|
“The biggest challenge in the adoption of any guideline is to change culture.”
|
|
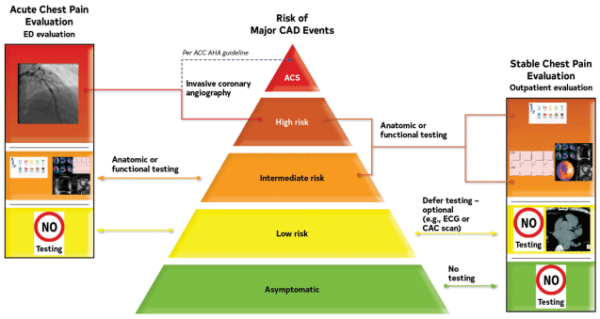
The Society for Cardiovascular Angiography and Intervention (SCAI) perspective on the new chest pain guidelines for cardiovascular imaging.
|
|
|
|
The major cardiac imaging societies weighed-in on the AHA/ACC’s new Chest Pain Guidelines, highlighting the notable shifts coming to cardiac imaging, and the adjustments they could require.
The cardiac CT and MRI societies took a victory lap, highlighting CCTA and CMR’s now-greater role in chest pain diagnosis, while forecasting that the new guideline will bring:
- Increased demand for cardiac CT & MR exams and scanners
- A need for more cardiac CT & MR staff, training, and infrastructure
- Requests for more cardiac CT & MR funding and reimbursements
- More collaborations across radiology, cardiology, and emergency medicine
The angiography and nuclear cardiology societies were less celebratory. Rather than warning providers to start buying more scanners and training more techs (like CT & MR), they focused on defending their roles in chest pain diagnosis, reiterating their advantages, and pointing out how the new guidelines might incorrectly steer patients to unnecessary or insufficient tests.
FFR-CT’s new role as a key post-CT diagnostic step made headlines when the guidelines came out, but the cardiac imaging societies don’t seem to be ready to welcome the AI approach. The nuclear cardiology and radiology societies called out FFR-CT’s low adoption and limited supporting evidence, while the SCCT didn’t even mention FFR-CT in its statement (and they’re the cardiac CT society!).
Echocardiography maintained its core role in chest pain diagnosis, but the echo society clearly wanted more specific guidelines around who can perform echo and how well they’re trained to perform those exams. That reaction is understandable given the sonographer workforce challenges and the expansion of cardiac POCUS to new clinical roles (w/ less echo training), although some might argue that echo AI tools might help address these problems.
The Takeaway
Imaging and shared decision-making play a prominent role in the new chest pain guidelines, which seems like good news for patient-specific care (and imaging department/vendor revenues), but it also leaves room for debate within the clinic and across clinical societies.
The JACC seems to understand that it needs to clear up many of these gray areas in future versions of the chest pain guidelines. Until then, it will be up to providers to create decision-making and care pathways that work best for them, and evolve their teams and technologies accordingly.
|




|
|
- CMR for Heart Transplant Monitoring: A team of Australian researchers found that cardiac MRI multiparametric mapping can effectively monitor patients for heart transplant rejection, suggesting that it could become an alternative to invasive endomyocardial biopsy (EMB). Analysis of 401 CMR and 354 EMB patients over a two-year follow-up period (40 w/ heart transplants) found that CMR accurately identified patients who would have rejected transplants (AUC: 0.92; NPV: 99%), while allowing 94% of patients to avoid EMBs (6% of CMR patients later received EMB).
- Gen Z Health Screenings: Researchers at the University of Michigan led a poll (n = 1,038) indicating that 81% of Gen Z patients want their providers to consider social factors such as food availability and education in health screenings. The survey also found that 25% want caregivers to provide resources regarding social needs, 22% want helpful general advice, and 11% want their provider to “just listen,” suggesting that younger generations are placing a larger emphasis on the SDOH factors impacting their day-to-day health.
- Us2.ai’s CE Mark and Multi-Modal Patent: Us2.ai continued its momentum, achieving its European CE Mark and a U.S. patent for using AI analysis of echo images combined with cardiac biomarkers for heart disease diagnosis. With its CE Mark, the Us2.v1 solution can now automate echo reporting in the US, Canada, Australia, New Zealand, Singapore, the UK, and 32 European countries. That’s quite a commercial presence for a company that was working on these approvals a year ago and just completed its Series A several months ago. It should also provide a major boost for Us2.ai’s ambitious global expansion plans.
- CT Contrast Pooling & Cardiac Arrest: A new study out of Taiwan confirmed that CT contrast agent pooling (CAP) should be flagged as a sign of imminent cardiac arrest with a higher risk of poor outcomes. The researchers reviewed chest and abdominal CTs from 128-consecutive emergency department patients who later experienced cardiac arrest (11 CTs w/ CAP), finding that CAP in CT scans predicted which patients would experience cardiac arrest within one hour with 86% accuracy (7.35 adjusted odds ratio) and patients with CAP had far lower survival rates (0% vs. 15.4%).
- DiA & ScImage: DiA Imaging Analysis announced a partnership with ScImage, combining its LVivo Seamless AI-based cardiac ultrasound analysis system with ScImage’s PICOM365 cloud enterprise imaging platform. LVivo Seamless selects the optimal cardiac ultrasound images and generates clinical indications of left and right ventricle function to aid clinician assessments. The alliance bolsters ScImage’s cardiac ultrasound capabilities, while further expanding DiA Imaging Analysis’ growing list of PACS and ultrasound partners (also: Change Healthcare, GE, Philips, Konica, Terason, SonoScape, Circle CVI, Watson Health).
- DLR Supports CT-FFR: A new study out of China found that deep learning image reconstruction (DLR) improves coronary CTA image quality, and more importantly, doesn’t negatively impact CT-FFR diagnostic performance. Using QFR and invasive FFR results as a reference, the authors analyzed DLR and four other reconstruction approaches using 182 CTAs from 33 patients, finding no significant difference across their CT-FFR values and diagnostic performance (AUCs: 0.83 vs. 0.81 to 0.86; p>0.05).
- Apple Watch Updates: Apple’s Worldwide Developer Conference brought significant health upgrades to the most popular wearable on the planet, with the Apple Watch receiving a new Medications feature that lets users track their medications, set up schedules / reminders, and view potential interactions from the Health app. Apple spent a majority of the Watch’s showcase discussing Medications and other health-related updates (AFib History, REM Sleep Tracking) as it looks to make health features a core component of the Watch’s value proposition.
- Hospital Margins Decline: Kauffman Hall’s latest National Hospital Flash Report shows that declining new patient visits and rising expenses contributed to the fourth consecutive month of negative hospital margins, which averaged -3.09% in April. System revenues fell 7% following a 5.7% month-over-month decline in patient days, reversing the modest rise in patient volumes recorded in March.
- UltraSight’s AI Guidance Evidence: An UltraSight and Sheba Medical Center pilot study demonstrated that UltraSight’s echo guidance AI software allows novice users to capture sonographer-level cardiac ultrasound images. After attending an 8-hour course, three medical assistants with no prior sonography training used UltraSight to perform echo exams on 61 patients, obtaining diagnostic-quality images with 100% of patients.
- Silent Atherosclerosis Evidence: The main results of the Miami Heart Study are officially published, confirming that many asymptomatic people have coronary plaque and stenosis that is detectable with low-dose coronary CT angiography. The researchers performed CCTAs on 2,359 asymptomatic individuals (53yr avg. age), finding that 49% had coronary plaque, 6% had at least moderate stenosis (≥50%), and 7% had plaques with at least one high-risk feature. CCTA also detected non-calcified plaque in 16% of patients with zero CAC scores (2.3% w/ high-risk plaques), demonstrating CCTA’s added value for risk stratification.
- FDA Can’t Regulate AI: A new opinion piece in PLOS Digital Health makes the case that AI’s pace of innovation is simply too quick for the FDA to regulate alone, and that a “distributed approach” is needed for proper regulation. The Harvard researchers argue that health systems should self-regulate by developing standards and training protocols that ensure AI tools are safe at the local level, while the FDA should only step in to regulate AI that’s intended for nationwide use or that has a high potential to negatively impact patient health.
|
|
|
|
|